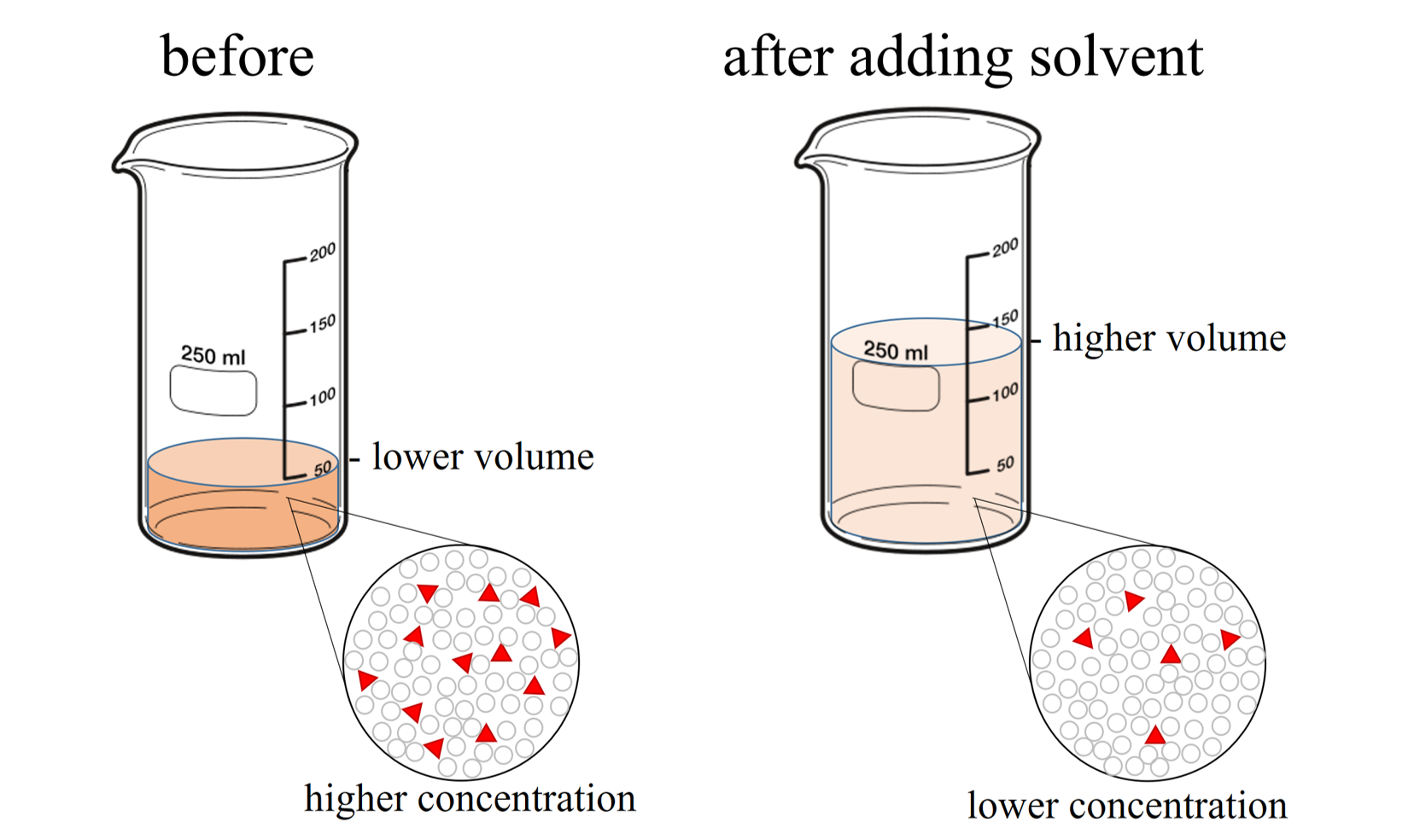
Dilution Method Definition . There are many ways of expressing concentration. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Of course, the resulting solution is. Explanations and examples of common methods. we will begin our discussion of solution concentration with two related and relative terms: For example, if you have a glass of. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard.
from chem.libretexts.org
Explanations and examples of common methods. Of course, the resulting solution is. There are many ways of expressing concentration. to dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard. we will begin our discussion of solution concentration with two related and relative terms: dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. For example, if you have a glass of.
14.7 Solution Dilution Chemistry LibreTexts
Dilution Method Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms: There are many ways of expressing concentration. Of course, the resulting solution is. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. For example, if you have a glass of. to dilute a solution means to add more solvent without the addition of more solute. Explanations and examples of common methods. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution Method Definition There are many ways of expressing concentration. Concentration is the removal of solvent, which. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Explanations and examples of common methods. For example, if you have a glass of. Of course, the resulting solution is. to dilute a solution means to add. Dilution Method Definition.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog Dilution Method Definition dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. There are many ways of expressing concentration. Of course, the resulting solution is. Concentration is the removal of solvent, which. For example, if you have a glass of. Explanations and examples of common methods. dilution is the process whereby the concentration. Dilution Method Definition.
From www.vrogue.co
Serial Dilution Method Definition Procedure Applicati vrogue.co Dilution Method Definition we will begin our discussion of solution concentration with two related and relative terms: Explanations and examples of common methods. Concentration is the removal of solvent, which. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the process of making a solution or scientific sample less concentrated. Dilution Method Definition.
From www.researchgate.net
Illustration of the agar dilution method the drug is the antimicrobial... Download Scientific Dilution Method Definition dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process. Dilution Method Definition.
From microbeonline.com
Broth Dilution Method for MIC Determination • Microbe Online Dilution Method Definition dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Of course,. Dilution Method Definition.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria • Microbe Online Dilution Method Definition Of course, the resulting solution is. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms: to dilute a solution means to add more solvent without the addition of more solute. For example, if you have a. Dilution Method Definition.
From www.researchgate.net
Agar dilution method with NPs. Download Scientific Diagram Dilution Method Definition we will begin our discussion of solution concentration with two related and relative terms: to dilute a solution means to add more solvent without the addition of more solute. For example, if you have a glass of. Concentration is the removal of solvent, which. dilution is the process of making a solution or scientific sample less concentrated. Dilution Method Definition.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry Dilution Method Definition we will begin our discussion of solution concentration with two related and relative terms: There are many ways of expressing concentration. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Concentration is the removal of solvent, which. dilution is the process whereby the concentration of a solution is lessened. Dilution Method Definition.
From exofldwwh.blob.core.windows.net
Dilution Method In Medicine at Joni Blomberg blog Dilution Method Definition There are many ways of expressing concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Explanations and examples of common methods. For example, if you have a glass of. Of course, the resulting. Dilution Method Definition.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Method Definition dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. we will begin our discussion of solution concentration with two related and relative terms: Explanations and examples of common methods. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal. Dilution Method Definition.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Method Definition There are many ways of expressing concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is. Concentration is the removal of solvent, which. For example, if you have a glass of. dilution is the process whereby the concentration of a solution is lessened by the. Dilution Method Definition.
From ceqnefyw.blob.core.windows.net
Dilution Method Of Ast at Clement Meador blog Dilution Method Definition There are many ways of expressing concentration. For example, if you have a glass of. Concentration is the removal of solvent, which. we will begin our discussion of solution concentration with two related and relative terms: Explanations and examples of common methods. dilution is the process whereby the concentration of a solution is lessened by the addition of. Dilution Method Definition.
From www.reddit.com
Serial Dilution in Microbiology Definition, Formula, Procedure and Calculator biology Dilution Method Definition For example, if you have a glass of. Explanations and examples of common methods. There are many ways of expressing concentration. to dilute a solution means to add more solvent without the addition of more solute. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of. Dilution Method Definition.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria • Microbe Online Dilution Method Definition Explanations and examples of common methods. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock. Dilution Method Definition.
From www.youtube.com
DILUTIONDEFINITION AND PRACTICE PROBLEMS YouTube Dilution Method Definition Explanations and examples of common methods. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Concentration is the removal of solvent, which. to dilute a solution means to add more solvent without the addition of more solute. For example, if you have a glass of. dilution is the addition. Dilution Method Definition.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Method Definition Of course, the resulting solution is. Explanations and examples of common methods. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. we will begin our discussion of solution concentration with two related and relative terms: Concentration is the removal of solvent, which. For example, if you have a glass of. . Dilution Method Definition.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Method Definition Of course, the resulting solution is. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Explanations and examples of common methods. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Concentration is the removal of solvent, which. dilution is the addition. Dilution Method Definition.
From dxorsyqnq.blob.core.windows.net
Dilution Adjective Definition at Robert Gonzales blog Dilution Method Definition we will begin our discussion of solution concentration with two related and relative terms: Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the addition. Dilution Method Definition.